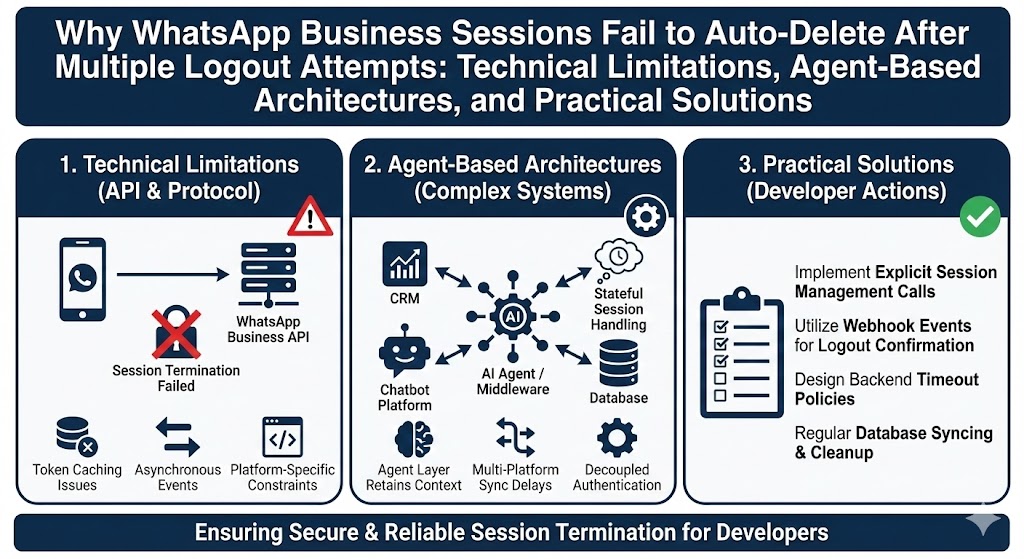
Introduction
The COVID-19 pandemic has been a defining global event, profoundly impacting public health, economies, and daily life. One of the most notable aspects of the pandemic has been the rapid development and deployment of vaccines. This article delves into the vaccine development process, the lessons learned from COVID-19, and how these insights can shape future vaccine research and public health strategies.
The Vaccine Development Process
- Traditional Vaccine Development
- Phases of Development: Traditional vaccine development typically involves several phases, including preclinical research, clinical trials (Phase 1, 2, and 3), and post-marketing surveillance. Each phase is designed to assess safety, efficacy, and optimal dosing.
- Vaccine Platforms: Conventional vaccine platforms include inactivated or killed virus vaccines, live attenuated vaccines, and subunit vaccines. Each platform has its own advantages and limitations, influencing the choice of approach for different diseases.
- Rapid Vaccine Development during COVID-19
- mRNA Vaccines: The COVID-19 vaccines developed by Pfizer-BioNTech and Moderna represent a novel approach using messenger RNA (mRNA). mRNA vaccines work by instructing cells to produce a protein that triggers an immune response, which has been shown to be highly effective in preventing COVID-19.
- Viral Vector Vaccines: The AstraZeneca-Oxford vaccine and the Johnson & Johnson vaccine use viral vectors to deliver genetic material encoding the SARS-CoV-2 spike protein. This approach has also proven effective in eliciting an immune response.
Lessons Learned from COVID-19 Vaccine Development
- Speed and Efficiency
- Accelerated Timelines: The COVID-19 vaccine development process was unprecedentedly rapid, with vaccines developed, tested, and authorized for emergency use within a year. This was achieved through unprecedented collaboration between governments, pharmaceutical companies, and research institutions.
- Streamlined Regulations: Regulatory agencies like the FDA and EMA adapted their processes to facilitate faster review and approval without compromising safety. Rolling reviews and accelerated pathways allowed for quicker deployment of vaccines.
- Global Collaboration and Resource Allocation
- International Partnerships: The pandemic highlighted the importance of global collaboration in vaccine research and distribution. Initiatives like COVAX aimed to ensure equitable access to vaccines across different countries, regardless of their economic status.
- Funding and Investment: Significant financial investments from governments and private entities enabled the rapid development and production of vaccines. This funding model demonstrated the potential for future large-scale public health responses.
- Vaccine Equity and Distribution
- Addressing Inequities: The pandemic exposed disparities in vaccine access and distribution, with lower-income countries facing significant challenges. Efforts to address these inequities, such as increasing vaccine production capacity and improving distribution infrastructure, are crucial for future health crises.
- Public Health Infrastructure: Strengthening global and national vaccine distribution networks is essential for ensuring timely and equitable access to vaccines. Lessons learned from the pandemic emphasize the need for robust logistics and supply chain management.
- Communication and Public Perception
- Combating Misinformation: The spread of misinformation about vaccines posed a significant challenge to public health efforts. Effective communication strategies, including transparent information and community engagement, are vital for building public trust in vaccines.
- Vaccine Hesitancy: Addressing vaccine hesitancy through education and outreach is crucial. Understanding and addressing the reasons behind vaccine reluctance can improve vaccine uptake and overall public health.
- Surveillance and Monitoring
- Ongoing Monitoring: Post-marketing surveillance has been critical for monitoring vaccine safety and efficacy. Continuous monitoring helps identify any adverse effects and ensures that vaccines remain safe and effective over time.
- Adapting to Variants: The emergence of new variants of SARS-CoV-2 highlighted the need for adaptable vaccine strategies. Booster doses and updated formulations may be necessary to address evolving viral strains.

Future Implications for Vaccine Development
- Advancements in Vaccine Technology
- Next-Generation Vaccines: Research into new vaccine technologies, such as nanoparticle vaccines and universal vaccines, holds promise for improving efficacy and broadening protection against various pathogens.
- Combination Vaccines: Combining vaccines for multiple diseases into a single formulation could simplify vaccination schedules and increase coverage.
- Pandemic Preparedness
- Rapid Response Frameworks: Establishing frameworks for rapid vaccine development and deployment can enhance preparedness for future pandemics. Lessons from COVID-19 can inform strategies for quicker responses to emerging infectious diseases.
- Global Collaboration Networks: Strengthening international collaborations and data-sharing networks will be essential for coordinated efforts in vaccine development and distribution during future health crises.
- Ethical and Policy Considerations
- Equity and Access: Ensuring equitable access to vaccines remains a critical challenge. Policymakers must address global health disparities and implement strategies to improve access for underserved populations.
- Ethical Considerations: Ethical issues, such as vaccine mandates and prioritization of certain groups, require careful consideration. Balancing public health needs with individual rights is a key aspect of vaccine policy.
Conclusion
The COVID-19 pandemic has been a pivotal moment in vaccine development, demonstrating the potential for rapid innovation and collaboration. The lessons learned from this experience offer valuable insights for future vaccine research and public health strategies. By building on these lessons, we can enhance our preparedness for future health challenges and improve global health outcomes.